In the evolving landscape of diabetes research, managing and analyzing vast amounts of data from diverse participants is crucial for advancing medical understanding. The challenge, however, lies in securely and efficiently exchanging, processing, and consolidating data from multiple sources—especially when different datasets exhibit varied patterns. surveilr provides an innovative solution to these challenges, transforming how diabetes data is handled in research studies.
The Role of surveilr in Diabetes Research Data Management
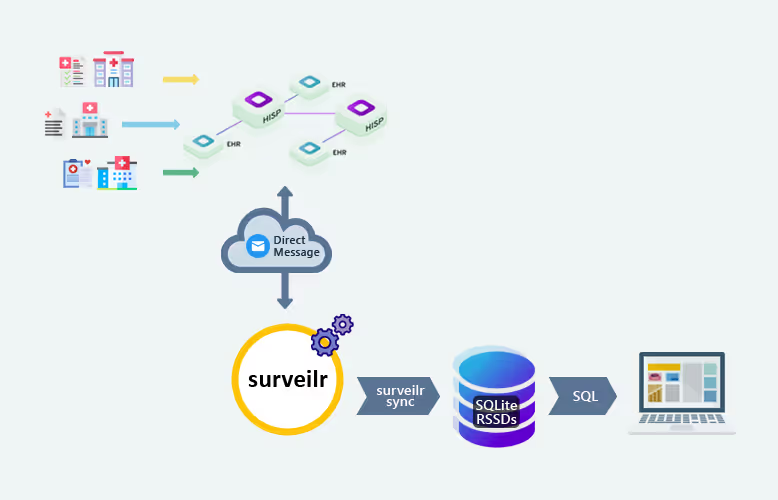
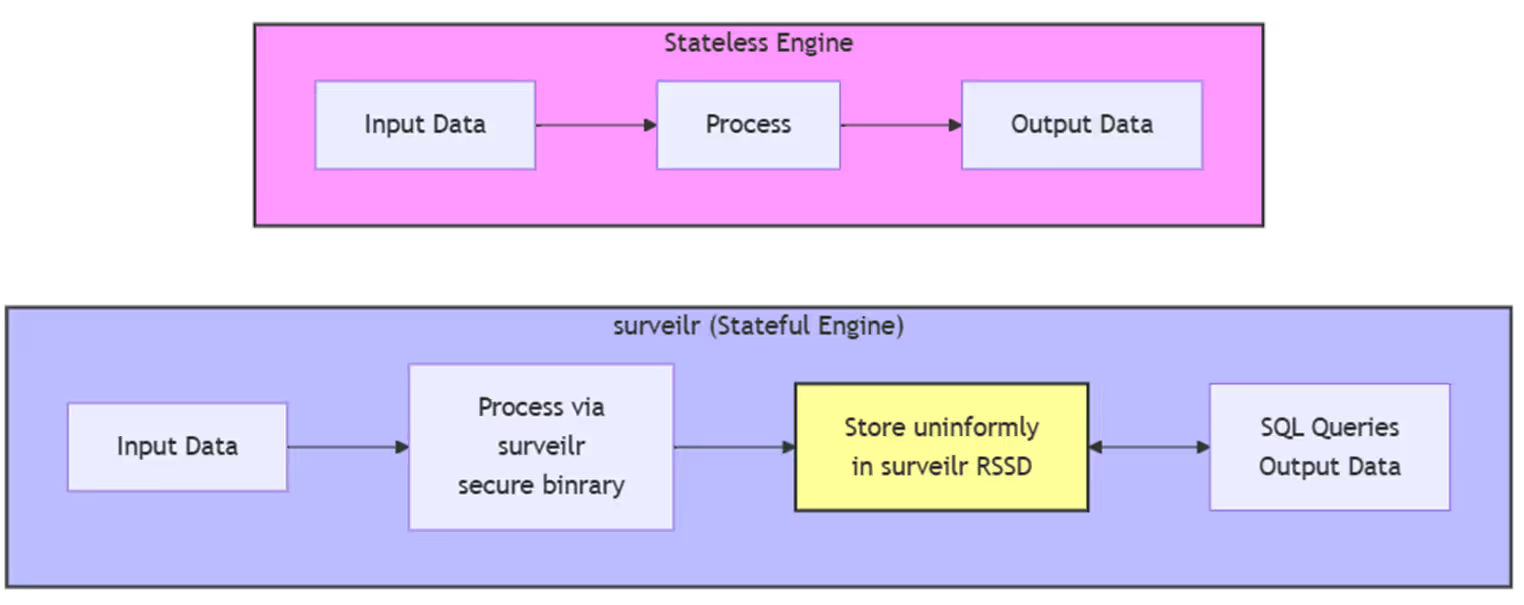
surveilr is a state-of-the-art platform designed to simplify data management in health-related research, specifically for studies involving complex datasets, like Continuous Glucose Monitoring (CGM) data. In diabetes research, these datasets often come from a variety of sources and participant profiles, requiring tools that can efficiently process and integrate the data into a unified format. By automating data ingestion, processing, and ensuring regulatory compliance, surveilr helps streamline these tasks, enabling researchers to focus on analysis and insights.
Challenges in Managing Diabetes Research Study Data
Managing diabetes research study data poses several unique challenges:
- Dataset Variability: Diabetes research datasets often differ in structure, with each dataset representing CGM data from different participants or study sites, which complicates data integration.
- Data Volume: The sheer volume of data generated in long-term diabetes studies is overwhelming. Processing this data efficiently and ensuring its accuracy is a critical task.
- Participant-Level Data: Diabetes studies typically involve tracking multiple participants, each with unique data points, such as CGM readings, blood glucose levels, and demographic information.
- Data Security and Privacy: Ensuring that sensitive patient information remains secure and compliant with privacy regulations such as HIPAA is a constant challenge.
How surveilr Solves Diabetes Research Data Challenges
surveilr addresses these challenges by providing a comprehensive suite of features designed to handle complex, large-scale datasets securely and efficiently.
1. Automated Data Processing and Integration
surveilr automates the ingestion and processing of diverse datasets, which might include CGM readings from different devices, demographic data, and clinical metrics. By validating the authenticity of the data sources and ensuring it is securely processed, surveilr saves researchers significant time and reduces the risk of human error.
2. Scalability for Large Datasets
Diabetes research generates vast amounts of data, which can overwhelm traditional data management systems. surveilr is designed to handle large-scale datasets seamlessly. It automatically processes and integrates the data, ensuring that even the largest datasets are handled without delays or performance issues.
3. Data Validation and Compliance
In diabetes research, ensuring the integrity and compliance of the data is paramount. surveilr ensures that all incoming data, including sensitive participant information, is processed in accordance with HIPAA and other regulatory standards. Additionally, surveilr can anonymize or de-identify data as necessary, ensuring privacy while maintaining the usefulness of the data for analysis.
4. Real-Time Data Processing
For diabetes research, real-time data availability is crucial, especially when analyzing patient responses to treatments or interventions. surveilr provides real-time processing capabilities, ensuring that data from participants is immediately available for analysis, facilitating faster insights.
5. Seamless Integration with Research Systems
surveilr integrates effortlessly with existing research infrastructure, including databases and other health information systems (HIS). Researchers can connect surveilr to their preferred platforms (e.g., PostgreSQL, EHR systems) to ensure that all data is synchronized and up-to-date, supporting collaborative research efforts.
6. Enhanced Security and Privacy
surveilr strengthens the security of diabetes research data through advanced encryption, automatic validation, and detailed audit trails. These measures protect sensitive health information from unauthorized access and ensure compliance with healthcare regulations.
Benefits of Using surveilr in Diabetes Research Studies
- Efficient Data Management:
surveilrautomates the processing of participant data, eliminating manual tasks and reducing the likelihood of errors. - Regulatory Compliance: Ensuring HIPAA compliance and data security is made simple with
surveilr’s built-in features for data anonymization and audit trails. - Seamless Data Integration:
surveilrsupports multiple data formats and integrates with various systems, ensuring smooth data flow across research platforms. - Scalability and Performance: Whether dealing with small or large datasets,
surveilrscales to meet the demands of diabetes research studies. - Enhanced Research Collaboration: By automating data handling and improving data accessibility,
surveilrfosters better collaboration among research teams.
Better Data Governance in Diabetes Research
- Centralized data repository for diabetes study information
- Enhanced audit trails for tracking data usage
- Simplified compliance reporting for research purposes
- Secure, localized data visualization for better study insights
Real-Time Data Processing
- Immediate availability of participant data for real-time analysis
- Fast integration with existing diabetes research databases and systems
- Minimizes delay in processing time for research updates
Robust Data Validation and Transformation
- Automated error detection and reporting for diabetes study data
- Validation capabilities for ensuring accurate research results
- Data anonymization for privacy protection and compliance
Conclusion
The complexities of managing diverse and voluminous data in diabetes research studies require efficient, secure, and compliant solutions. surveilr offers an invaluable tool for diabetes research teams by automating data processing, ensuring regulatory compliance, and providing seamless integration with existing research systems. By enhancing data management workflows, surveilr helps researchers focus on advancing medical knowledge and improving patient care, all while reducing administrative overhead and ensuring the privacy and integrity of the data.